Let's talk chemistry and electricity. Most motorcycles can't operate without a power source, also known as the battery or battery pack.
This system consists of multiple cells (often called accumulators), each producing about 2.1 volts. Scooters and small motorcycles generally use a 6-volt battery pack (3 cells). In the case of a 12-volt battery, we're talking twice as many cells.
A battery pack supplies power (amps) at a constant density (volts) or high-intensity current at ultra-low temperatures. It feeds the starter motor and the ignition system.
Also, the battery pack supplies power to various lights and accessories connected to the power circuit. This is especially important when the combustion engine is idling or shut down.
The most commonly used battery pack is the lead-acid type. Of course, there are many other types, like nickel or hydrogen batteries. However, they're less popular due to their higher costs.
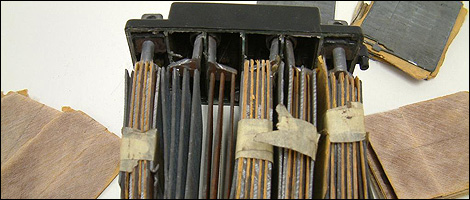 |
| Battery accumulators |
Battery packs are made up of several interlinked cells which lay in a non-conductive substance. Each cell has several lead plates wired in parallel. There are two types of plates: positive (+) and negative (-). The parallel layout increases the active surface, which has a direct impact on the intensity of the current. There's always an additional negative plate for each cell in order to protect the positive plates which are less rigid.
To prevent a short circuit, a separator (made of paper, cellulose, plastic or fiberglass) is placed between the lead plates. This isolating material is shaped like a fan to facilitate the contact of acid with the plates. It also allows the battery pack to hold a greater amount of acid.
The grids acting as the backbones of the plates are made of a lead-antimony alloy for superior rigidity. However, antimony creates an electrochemical reaction which accelerates self-discharge. That's why it is currently being replaced by calcium, for example. Once they're covered with an active paste, the grids become plates. Said paste is made of 99.9-percent pure lead (as oxidized powder). Specific additives are incorporated to polarize the compound. Once the plates are "baked" in a specially-designed oven, engineers measure their thickness before stocking them for oxidation.
Dry batteries are different: the cells are put in trays with miniscule amounts of acid (into which the current flows). Then, lead protoxide of the positive plates turns into lead peroxide, while lead protoxide of the negative plates transforms into pure lead. At this stage, they're extremely difficult to manipulate since they can catch fire when contacting ambient air.
As soon as the plates are finalized, they're put into the tray, which is usually made of polypropylene (about 60-percent lighter than ebonite). This tray needs to withstand violent shocks. Each battery cell features a small blowhole to evacuate the pressure generated by the heat of the chemical reactions when the battery is "on". A lid on top of the tray ensures that the unit is completely sealed. You'll also find filling caps on traditional battery packs, which use a specially-formulated mixture of water and acid. Don't try to make your own mixture because the risk of an explosion is pretty high.
Photo Credit : Henri Lebarbé
